Covid Antivirals Direct From Manufacturer

Manufacturer Direct
CIF Airfreight Pharmaceutical or Government Buyers
Within 48 hours of Buyer document submission, AllVaxx issues FCO and assigns specific antiviral lots to Buyer:
a. WHO Prequalification document
b. Certificate of Pharmaceutical Product & Certificate of Origin
c. GMP Certificate
d. Certificate of Analysis
e. Declaration of Conformity
SGS inspectors secure the loads for shipping.
With easy payment via International 3rd Pary Escrow antivirals are loaded on cargo planes for immediate delivery.
Please Note: Covid Antiviral supply is subject to Emergency Use Authorization (EUA) of Covid-19 Antiviral treatment by the Importing country government. Availability based on limited ready stock, logistics, and government regulations.
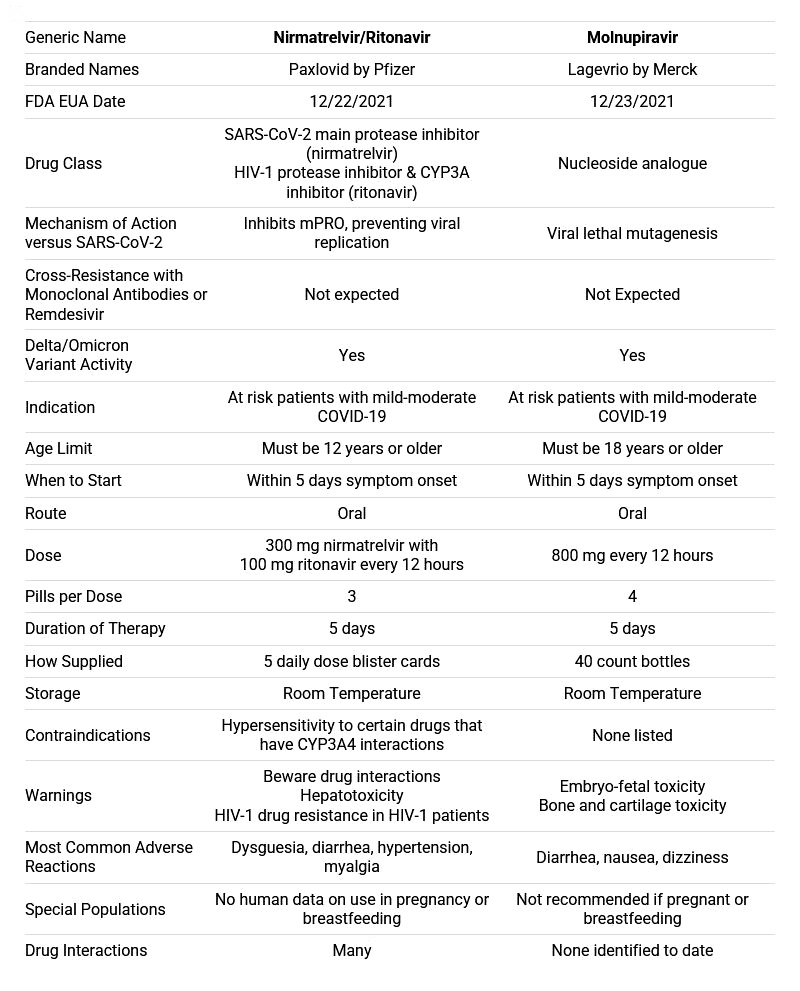
MERCK - Molnupiravir/Lagevrio
Authorized Generics by Cipla, Dr Reddy, MSN, Sun Pharma, Aurobindo, and Torrent Pharma
Merck was FDA authorized for emergency use in the U.S. December 23rd 2021.
Status: Emergency use in the U.S and other countries, including in the European Union and other countries.
Molnupiravir is an investigational oral antiviral drug that inhibits the multiplication of the infection-causing virus, thereby decreasing the viral load in the body.
Recommended for: Adults 18 and older. Not available for children or pregnant women.
Dosage: 4 200mg pills/day, for 5 days. Total 40 pills/treatment.
To date, Merck has shipped Molnupiravir to 14 countries; in countries where it is approved or authorized, patients have begun to receive the drug.


Clinical Trials
As a single oral medicine that can be taken at home, early treatment with molnupiravir significantly reduced the risk of hospitalization or death in patients at high risk for progressing to severe COVID-19. Importantly for patients, there were markedly fewer deaths among those taking molnupiravir in clinical study. Molnupiravir is a critical addition to the measures available to help curb the impact of COVID-19 on patients, healthcare systems and public health.
Merck embraces the responsibility to bring this important medicine forward to patients globally as quickly as possible. They are confident in the promise of molnupiravir as a medicine that can be taken at home with no known drug-drug interactions and believe it will have a positive impact as part of the global effort to fight the COVID-19 pandemic.

Pfizer - Paxlovid
COMING SOON
The US Food and Drug Administration issued an emergency use authorisation (EUA) for Pfizer’s Paxlovid for the treatment of mild-to-moderate Covid-19 in patients over 12 and weighing at least 40 kilos.
Paxlovid is a combination of 2 drugs — nirmatrelvir and ritonavir. Both drugs are known as protease inhibitors. Nirmatrelvir and ritonavir bind with specific proteases in the coronavirus and inhibits their functions.
The drug is approved for those at high risk of progressing to severe Covid-19, including hospitalization or death. Paxlovid is a prescription administered as soon as possible after diagnosis of Covid-19 and within 5 days of symptom onset.
Paxlovid is administered as 3 tablets — 2 tablets of nirmatrelvir and 1 tablet of ritonavir — taken together, orally, twice daily for 5 days, for a total of 30 tablets.
While there are some possible side-effects of the drug, and it can adversely impact the functioning of some other drugs (if taken together), the US Food and Drug Administration concluded that the benefits of Paxlovid outweigh its possible risks.
AllVax is here to meet all your antiviral/vaccine needs
Call/Whatsapp Travis Luedke:
+1 210 314 0586
© 2020 AllVaxx.com All Rights Reserved

