Covid Vaccines Direct From Manufacturer

10 Million Vaccines/Airplane
Within 72 hours from Buyer document submission, funds verification and deposit, vaccine allocation lot numbers are assigned to the Buyer:
a. WHO Prequalification document
b. Certificate of Pharmaceutical Product & Certificate of Origin
c. GMP Certificate
d. Certificate of Analysis
e. Declaration of Conformity
SGS inspectors secure the loads for shipping.
With easy payments via 3rd party escrow program, vaccines are loaded on cargo planes within 48 hours of escrow payment release.
Please Note: Vaccine supply is subject to Emergency Use Authorization (EUA) of Covid-19 vaccines by the Importing country government. Vaccine availability is based on limited ready stock, logistics capabilities, and government regulations.
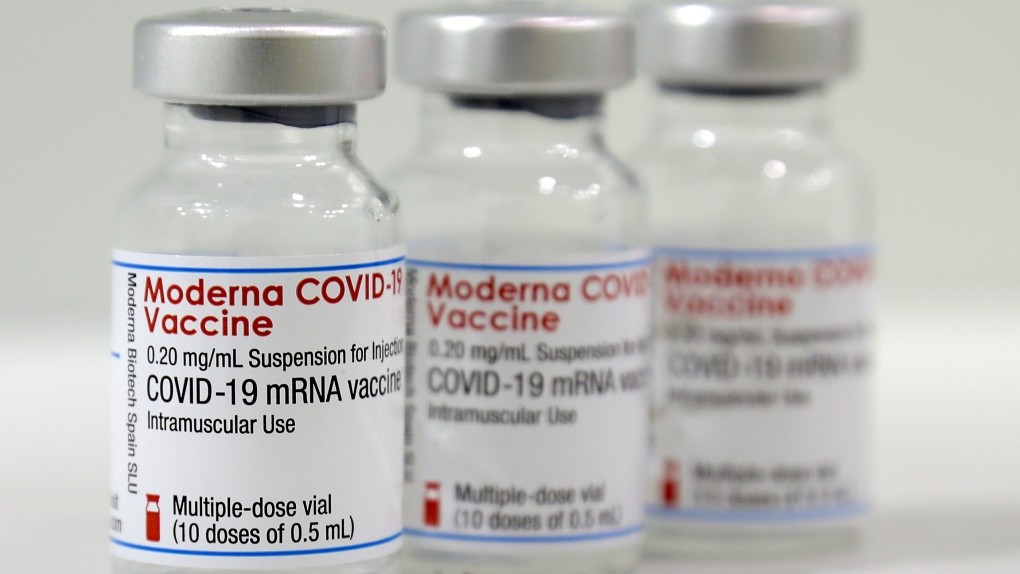
Moderna - Spikevax
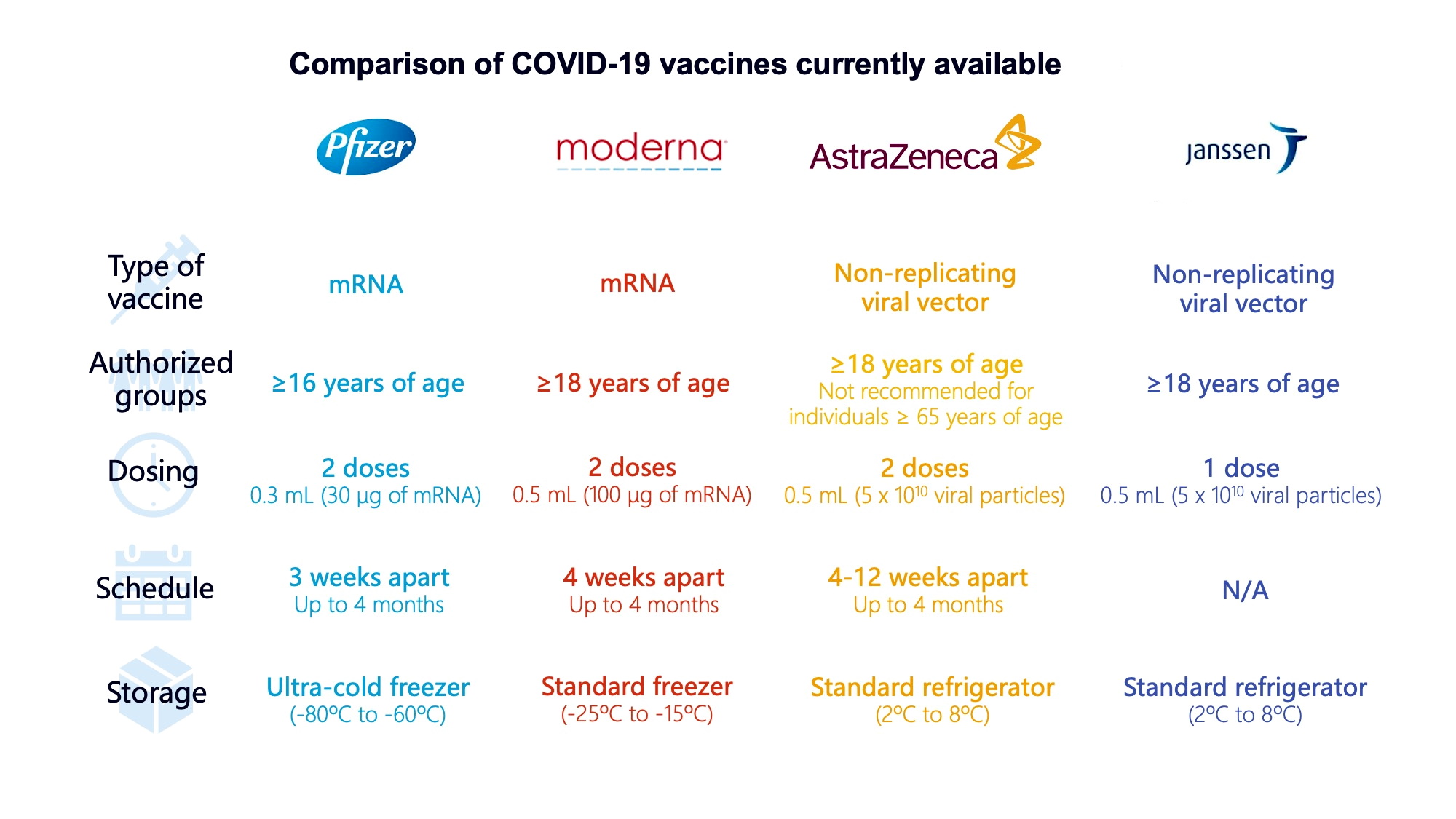
Moderna’s vaccine was authorized for emergency use in the U.S. about a week after the Pfizer vaccine. Moderna uses the same mRNA technology as Pfizer and has a similarly high efficacy at preventing symptomatic disease. It also needs to be stored in freezer-level temperatures.
Status: Emergency use in the U.S and other countries, including in the European Union (it’s been approved in Switzerland) and other countries.
Recommended for: Adults 18 and older. While the vaccine is not yet available for children, the company says its vaccine provides strong protection for children as young as 12, and it is testing its efficacy for children ages 5-11.
Dosage: Two shots, 28 days apart, fully effective two weeks after the second dose.

Pfizer - BioNTech - Comirnaty
On December 11, 2020, this became the first COVID-19 vaccine to receive a Food and Drug Administration (FDA) Emergency Use Authorization (EUA), after the company reported positive initial clinical trial data that showed the vaccine was highly effective at preventing symptomatic disease. This is a messenger RNA (mRNA) vaccine, which uses a relatively new technology. It must be stored in freezer-level temperatures, which can make it more difficult to distribute than some other vaccines.
Pfizer also is the first company to submit a request to the FDA for full approval of its vaccine. In addition, it is seeking FDA authorization for a third dose, or booster dose, of its original vaccine, and says it is planning to start clinical trials in August to test a booster shot against the Delta variant.
Status: Emergency use in the U.S. and other countries, including in the European Union (under the name Comirnaty).
Recommended for: Anyone 12 or older. The vaccine is being studied in children ages 5-11.
Dosage: Two shots, 21 days apart; fully effective two weeks after second shot.
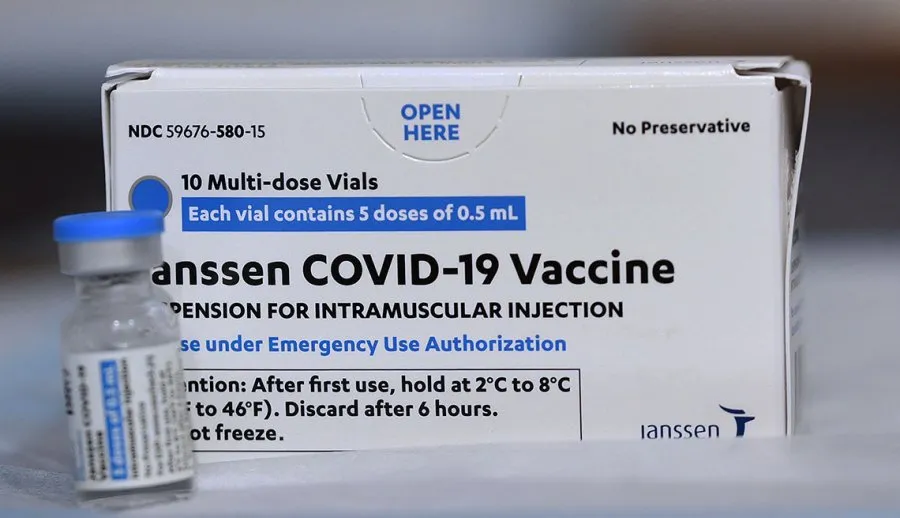
Johnson & Johnson - Jansen - Jycovson - Jcovsen - Jycovden
The FDA granted emergency use authorization for Johnson & Johnson’s vaccine in February 2021. In November of 2020, Johnson & Johnson announced it would launch a second Phase 3 clinical trial to study using two doses, two months apart, to see if that regimen will provide better protection. Unlike the Pfizer and Moderna vaccines, this is a carrier, or virus vector, vaccine. It can be stored in normal refrigerator temperatures, and because it requires only a single shot, it is easier to distribute and administer.
Status: Emergency use in the U.S. and other countries, including in the European Union (under the name Janssen).
Recommended for: Adults 18 and older.
Dosage: Single shot. Fully effective two weeks after vaccination.
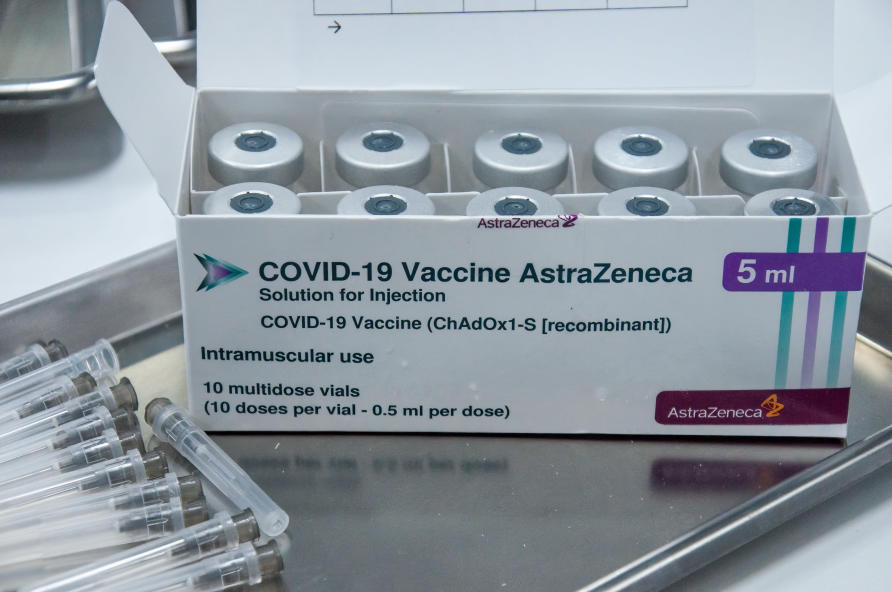
Oxford - AstraZeneca - Vaxzevria
This vaccine, which is currently being distributed in the United Kingdom and other countries, is distinguished from some of its competitors by its lower cost—it’s cheaper to make per dose, and while some of the other vaccines must be stored frozen, this one can be stored in normal refrigeration for at least six months, making it easier to distribute.
Oxford-AstraZeneca is currently studying the efficacy of a booster shoot.
Status: Not available in the U.S., authorized for emergency use in other countries, including in the European Union (under the name Vaxzevria) and the United Kingdom.
Recommended for: Adults 18 and older
Dosage: Two doses, four to 12 weeks apart.
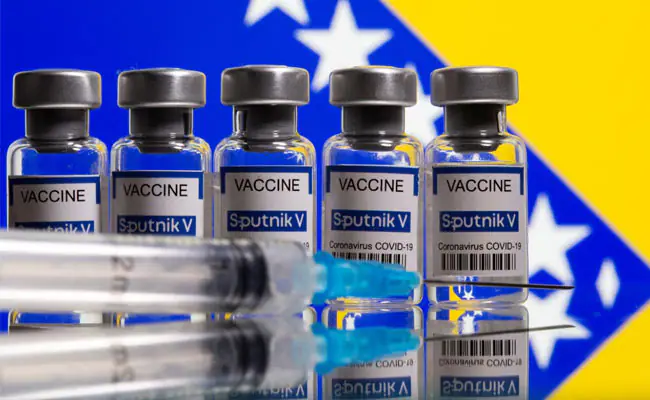
Sputnik V - Gamaleya
Sputnik V is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It was initially approved for distribution in Russia and then in 59 other countries (as of April 2021).
Emergency distribution of the vaccine began in December 2020. By February 2021 over a billion doses of the vaccine had been ordered for immediate distribution worldwide.
Effectiveness: On 29 June 2021, the director of the Gamaleya Institute, Denis Logunov, reported that Sputnik V is about 90% effective against the Delta variant.
The two doses are separated by three weeks, rather than the 8-12 weeks usually recommended for the Oxford-AstraZeneca vaccine.
Sputnik V doesn’t require the ultra-cold temperatures like the mRNA-based vaccines, which makes it an attractive candidate for many countries desperate for vaccines.
AllVax is here to meet all your vaccine needs
Call/Whatsapp Travis Luedke:
+1 210 314 0586
© 2020 AllVaxx.com All Rights Reserved

